Charcoal and Activated Carbons
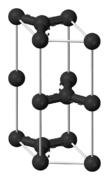
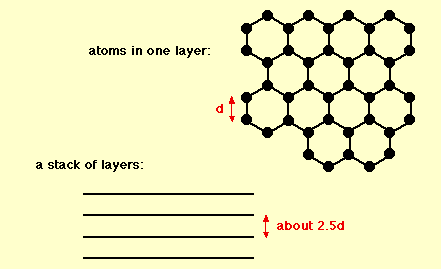
Graphite
The chemical bonds in graphite are similar in strength to those found in diamond. However, the lattice structure of the carbon atoms contributes to the difference in hardness of these two compounds; graphite contains two dimensional lattice bonds, while diamond contains three dimensional lattice bonds. The carbon atoms within each layer of graphite contain weaker intermolecular bonds. This allows the layers to slide across each other, making graphite a soft and malleable material.
Various studies have demonstrated that graphite is an excellent mineral with several unique properties. It conducts heat and electricity and retains the highest natural strength and stiffness even in temperatures exceeding 3600°C. This material is self-lubricating and is also resistant to chemicals.
Although there are different forms of carbon, graphite is highly stable under standard conditions. Depending upon its form, graphite is utilized for a wide range of applications.
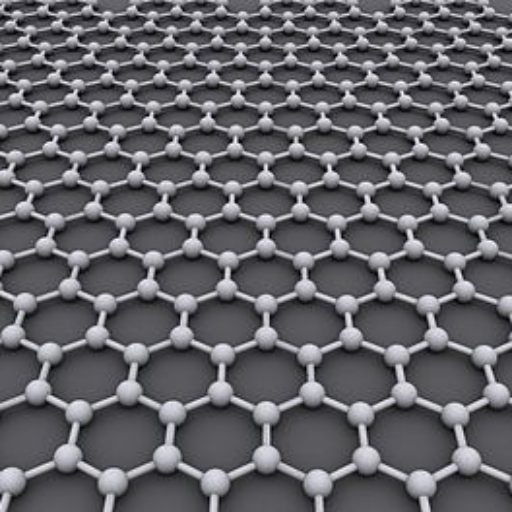
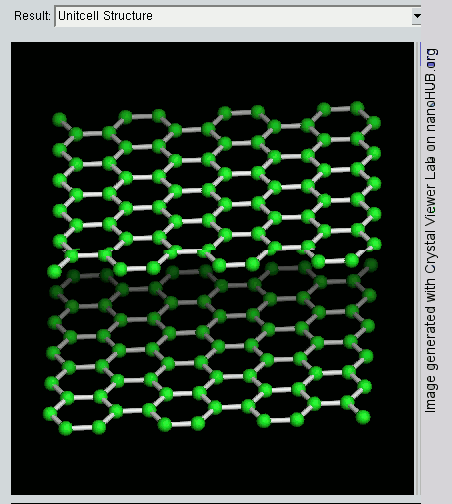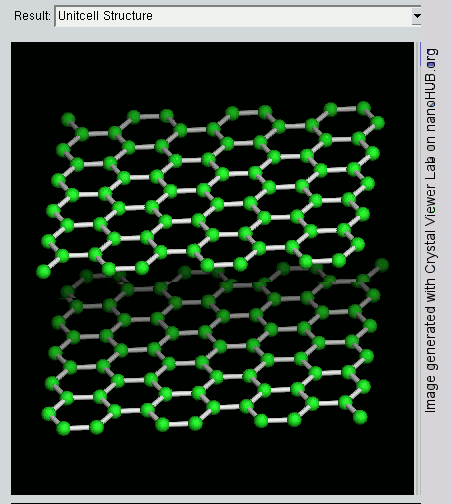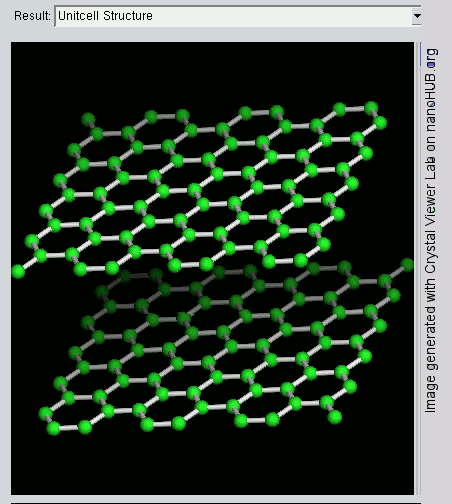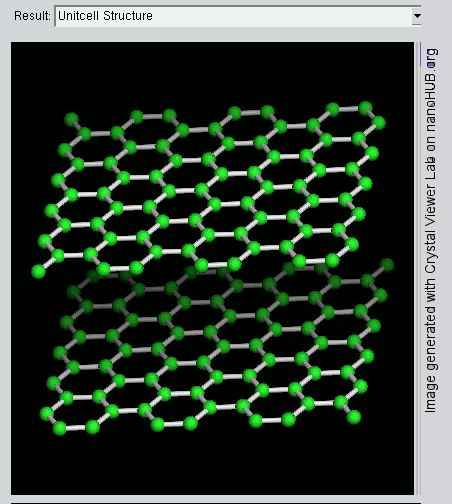
Graphene
What is the Difference between Graphite and Graphene ?
Since graphite has a planar structure, its electronic, acoustic, and thermal properties are highly anisotropic. This means, phonons pass much more easily along the planes than they do when trying to pass via the planes. However, graphene has very high electron mobility and, like graphite, is a good electrical conductor, due to the occurrence of a free pi (p) electron for each carbon atom.
However, graphene has much higher electrical conductivity than graphite, due to the occurrence of quasiparticles, which are electrons that function as if they have no mass and can travel long distances without scattering. In order to fully realize this high level of electrical conductivity, doping needs to be carried out to overcome the zero density of states which can be visualized at the Dirac points of graphene.
Fullerence
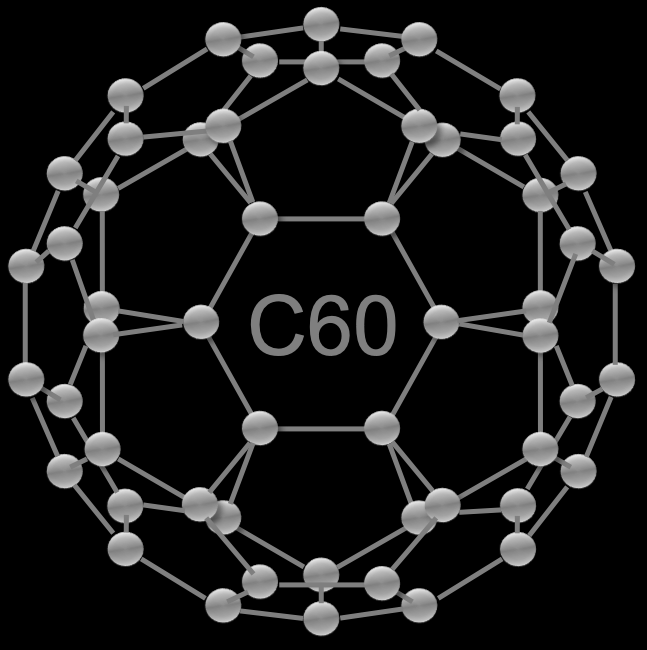
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene (isolated atomic layers of graphite), which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family.
Fullerenes with a closed mesh topology are informally denoted by their empirical formula Cn, often written Cn, where n is the number of carbon atoms. However, for some values of n there may be more than one isomer.
The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball of association football (“soccer”). Nested closed fullerenes have been named bucky onions. Cylindrical fullerenes are also called carbon nanotubes or buckytubes. The bulk solid form of pure or mixed fullerenes is called fullerite.
Fullerenes had been predicted for some time, but only after their accidental synthesis in 1985 were they detected in nature and outer space. The discovery of fullerenes greatly expanded the number of known allotropes of carbon, which had previously been limited to graphite, diamond, and amorphous carbon such as soot and charcoal. They have been the subject of intense research, both for their chemistry and for their technological applications, especially in materials science, electronics, and nanotechnology.