Graphite, archaically referred to as plumbago, is a crystalline allotrope of carbon, a semimetal, a native element mineral, and a form of coal. Graphite is the most stable form of carbon under standard conditions. Therefore, it is used in thermochemistry as the standard state for defining the heat of formation of carbon compounds. Graphite and diamond are the two mineral forms of carbon. Diamond forms in the mantle under extreme heat and pressure. Most graphite found near Earth’s surface was formed within the crust at lower temperatures and pressures. Graphite and diamond share the same composition but have very different structures. Graphite and diamonds are the only two naturally formed polymers of carbon. Graphite is essentially a two dimensional, planar crystal structure whereas diamonds are a three dimensional structure. Graphite is an excellent conductor of heat and electricity and has the highest natural strength and stiffness of any material. It maintains its strength and stability to temperatures in excess of 3,600°C and is very resistant to chemical attack. At the same time it is one of the lightest of all reinforcing agents and has high natural lubricity.
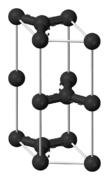 Graphite has a layered, planar structure. The individual layers are called graphene. In each layer, the carbon atoms are arranged in a honeycomb lattice with separation of 0.142 nm, and the distance between planes is 0.335 nm. Atoms in the plane are bonded covalently, with only three of the four potential bonding sites satisfied. The fourth electron is free to migrate in the plane, making graphite electrically conductive. However, it does not conduct in a direction at right angles to the plane. Bonding between layers is via weak van der Waals bonds, which allows layers of graphite to be easily separated, or to slide past each other.
Graphite has a layered, planar structure. The individual layers are called graphene. In each layer, the carbon atoms are arranged in a honeycomb lattice with separation of 0.142 nm, and the distance between planes is 0.335 nm. Atoms in the plane are bonded covalently, with only three of the four potential bonding sites satisfied. The fourth electron is free to migrate in the plane, making graphite electrically conductive. However, it does not conduct in a direction at right angles to the plane. Bonding between layers is via weak van der Waals bonds, which allows layers of graphite to be easily separated, or to slide past each other.
The two known forms of graphite, alpha (hexagonal) and beta (rhombohedral), have very similar physical properties, except for that the graphene layers stack slightly differently. The alpha graphite may be either flat or buckled. The alpha form can be converted to the beta form through mechanical treatment and the beta form reverts to the alpha form when it is heated above 1300 °C.
Graphite is a mineral that forms when carbon is subjected to heat and pressure in Earth’s crust and in the upper mantle. Pressures in the range of 75,000 pounds per square inch and temperatures in the range of 750 degrees Celsius are needed to produce graphite. These correspond to the granulite metamorphic facies.
What is graphite used for?
Natural graphite is mostly consumed for refractories, batteries, steelmaking, expanded graphite, brake linings, foundry facings and lubricants. Graphene, which occurs naturally in graphite, has unique physical properties and is among the strongest substances known. However, the process of separating it from graphite will require more technological development.
What is the Structures of graphite ?
Graphite has a layer structure which is quite difficult to draw convincingly in three dimensions. The diagram below shows the arrangement of the atoms in each layer, and the way the layers are spaced.
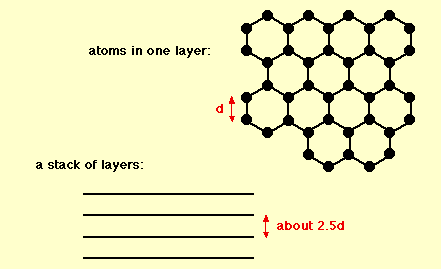
Notice that you can’t really draw the side view of the layers to the same scale as the atoms in the layer without one or other part of the diagram being either very spread out or very squashed.
In that case, it is important to give some idea of the distances involved. The distance between the layers is about 2.5 times the distance between the atoms within each layer.
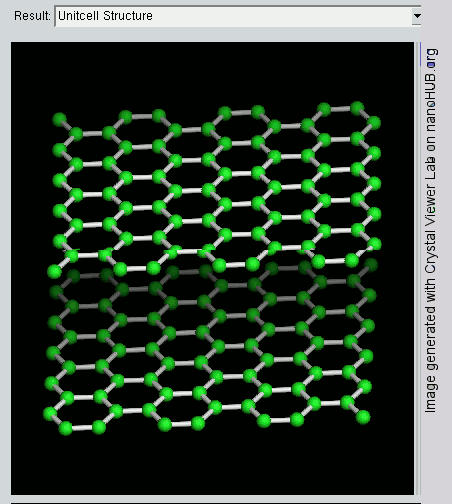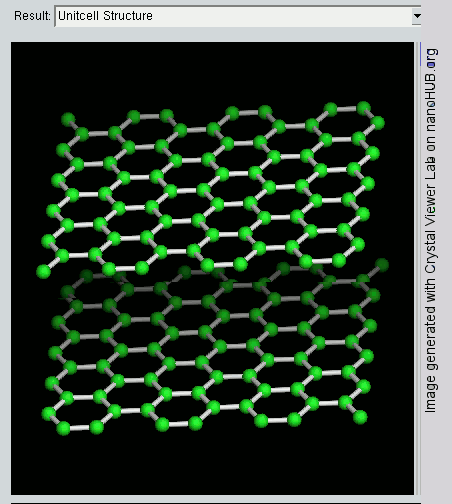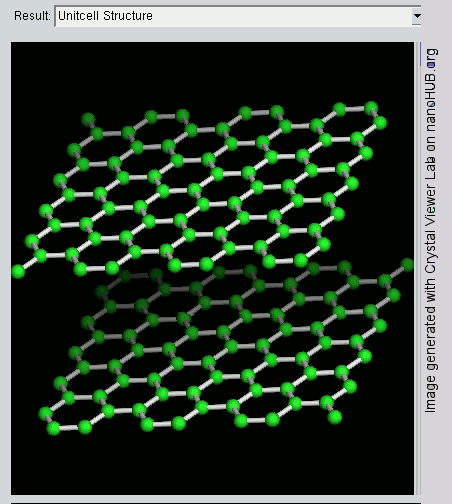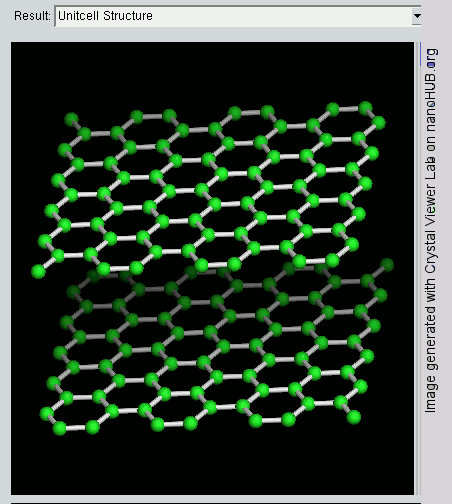
The layers, of course, extend over huge numbers of atoms – not just the few shown above.
You might argue that carbon has to form 4 bonds because of its 4 unpaired electrons, whereas in this diagram it only seems to be forming 3 bonds to the neighboring carbons. This diagram is something of a simplification, and shows the arrangement of atoms rather than the bonding.
What is The properties of graphite ?
- has a high melting point, similar to that of diamond. In order to melt graphite, it isn’t enough to loosen one sheet from another. You have to break the covalent bonding throughout the whole structure.
- Graphite’s high thermal stability and electrical and thermal conductivity facilitate its widespread use as electrodes and refractories in high temperature material processing applications.
- has a soft, slippery feel, and is used in pencils and as a dry lubricant for things like locks. Graphite and graphite powder are valued in industrial applications for their self-lubricating and dry lubricating properties. You can think of graphite rather like a pack of cards – each card is strong, but the cards will slide over each other, or even fall off the pack altogether. When you use a pencil, sheets are rubbed off and stick to the paper.
- has a lower density than diamond. This is because of the relatively large amount of space that is “wasted” between the sheets.
- is insoluble in water and organic solvents – for the same reason that diamond is insoluble. Attractions between solvent molecules and carbon atoms will never be strong enough to overcome the strong covalent bonds in graphite.
- conducts electricity. Graphite is an electric conductor, consequently, useful in such applications as arc lamp electrodes. The de-localized electrons are free to move throughout the sheets. If a piece of graphite is connected into a circuit, electrons can fall off one end of the sheet and be replaced with new ones at the other end.

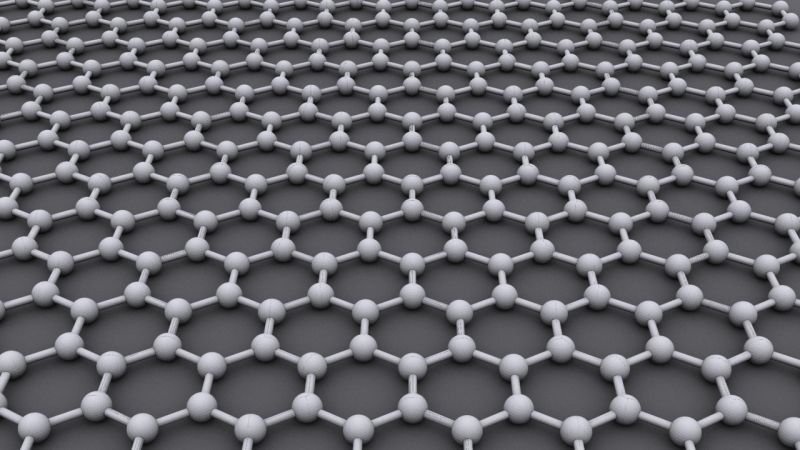





 Graphite has a layered, planar structure. The individual layers are called
Graphite has a layered, planar structure. The individual layers are called 
